News & Insights
Besides sharing our hands-on expertise in projects, we regularly share industry insights, updates, and news. At NecstGen, we believe in sharing best practices to advance patient health. Together.






News
Stay up to date with the latest news, press releases and events here!
Accelerating the Translation of iPSCs from Research to Clinical Application
An Interview with Catalina Gomez-Puerto, Senior Scientist
Webinar: Scale-up Principles for Viral Vector Production
Register now for our upcoming webinar to learn the principles for robust, high-yield viral vector production!
NecstGen Appoints Rick Hibbert as CEO
NecstGen welcomes Dr. Rick Hibbert as CEO from 1 Oct 2025. An experienced biopharma leader, Rick will drive our mission to advance Cell and Gene Therapies from research to patients worldwide.
The CGT Circle: Leiden
The CGT Circle is a community by women for women and this free of charge networking event is about connecting women within the Cell & Gene Therapy ecosystem.
NecstGen Partners with World Marrow Donor Association
NecstGen and WMDA are partnering to accelerate access to life-saving cell and gene therapies. This collaboration unites two non-profits committed to patient and donor safety, combining manufacturing expertise with a global donor network.
TechTalk XL 2025: Cell & Gene Therapies
As part of Dutch Bio Science Week 2025, TechTalkXL brought together the Cell and Gene Therapy community for an afternoon of focused exchange at Mirai House. Organised by NecstGen in collaboration with Leiden Bio Science Park, the event provided a setting to examine...
NecstGen and Astraveus Collaborate to Evaluate Benchtop Cell Factory™ Technology for Scalable CAR-T Manufacturing
NecstGen and Astraveus have joined forces to evaluate the Lakhesys™ Benchtop Cell Factory—a novel microfluidic platform designed to streamline and scale CAR-T manufacturing.
Webinar Announcement: Improving lentiviral vector yield with 2G UNic technology
Join NecstGen and ProteoNic for our webinar “Improving lentiviral vector yield with 2G UNic technology”, presented by Maurice van der Heijden (Director of R&D, ProteoNic) and Diederik Lokhorst (Viral Vector Scientist, NecstGen). Discover more about ProteoNic’s 2G...
TechTalk XL – Cell & Gene Therapies – Dutch Bio Science Week 2025
NecstGen is pleased to host the next edition of TechTalk XL during the Dutch Bio Science Week 2025 this summer. We invite you to join us for a focused afternoon of content and conversation on Cell & Gene Therapies.
NecstGen Celebrates Paul Bilars’ Five Years of Leadership
After more than five years of dedicated leadership, Paul Bilars will step down as CEO of NecstGen in July. Paul played a pivotal role in founding NecstGen and establishing it as a center of excellence for Cell and Gene Therapy.
NecstGen Hosts bioMérieux QC User Group Meeting in Leiden
Last Thursday, NecstGen had the pleasure of hosting the bioMérieux Pharma Quality Control User Group meeting “Automation and Digitalisation in Your QC Micro Lab” in Leiden, in close collaboration with the bioMérieux team. The event brought together users of bioMérieux...
Exploring the US Cell Therapy Market with Georgia Tech MBA Students
NecstGen is a mission-driven CDMO, operating as a non-profit organization to challenge the high costs of CGT therapies. From our cutting-edge facility located in Leiden, The Netherlands, we are able to serve clients all over the world, including US-based companies....
Welcoming Fraunhofer IZI at NecstGen
It was great to welcome Prof. Ulrike Köhl, Director of the Fraunhofer Institute for Cell Therapy and Immunology, and Paul Franz from Fraunhofer IZI to NecstGen! Over the past two days, we provided a facility tour and had inspiring discussions on potential...
NecstGen visits BIA Separations Inc./Sartorius in Slovenia
Last week, Diederik Lokhorst and Cristina Muñoz Lopez from NecstGen visited BIA Separations Inc./Sartorius in Slovenia for hands-on training in chromatography and analytical tools. This visit provided valuable insights into monolith technology and the PATfix...
RegMed Forum in Berlin
Today Sophia Kolbe attended the RegMed Forum in Berlin, where discussions focused on the critical challenges of process development, GMP readiness, and clinical translation of Advanced Therapy Medicinal Products (ATMPs). With Europe catching up in early-phase ATMP...
Webinars
At NecstGen, we are dedicated to driving innovation and knowledge-sharing in the fields of gene and cell therapy. Our webinar series is designed to provide in-depth insights into cutting-edge topics, including CAR T cell therapies and induced pluripotent stem cells (iPSCs), two of the most promising areas in modern medicine.
Webinar Improving Lentiviral Vector Yield with 2G UNic™ Technology
Improving Lentiviral Vector Yield with 2G UNic™ Technology Lentiviral vectors (LVs) are indispensable for ex vivo gene therapies, but producing them at scale while maintaining high quality and yield remains a major challenge. In this expert-led webinar, you’ll learn...
iPSC Differentiation for Curative Regenerative Cell Therapies of the Future
Learn how induced pluripotent stem cells (iPSCs) are being developed into potentially life-changing therapies.
Webinar Reducing Lentivirus Costs
Discover how our Design of Experiments (DOE) methodology optimises cost-efficiency in GMP manufacturing to turn a refined lab process into a scalable, GMP-ready protocol.
Webinar LV Process for CAR T
Shifting from traditional, ad hoc methodologies to a structured, data-based development approach to create a scalable, GMP-ready process.
Webinar CAR Therapy Today & Tomorrow
Explore available CAR therapies, differentiation, development challenges, and lessons learned from our expert scientist.
Webinar Product Quality for Cell Therapies
This session is all about building and testing strategies to set the groundwork for effective development and testing in your own work.
Webinar iPSC Banking & Derivation
iPSC Banking & Derivation Ever wondered how to transition from bench to GMP suitable processes in the iPSC-derived therapies field? Yes? Watch the webinar replay on iPSC Derivation & Banking.Key areas of focus include: Transitioning from Bench to GMP Suitable...
Stem Cells
Stem cells hold enormous potential for advancing regenerative medicine, with the ability to repair damaged tissues, treat degenerative diseases, and model complex conditions.
iPSC Reprogramming & Transdifferentiation
iPSC reprogramming and cell transdifferentiation offer exciting cellular science opportunities. Learn how these approaches are changing regenerative medicine and cellular therapy.
Differentiation of Human iPSCs
Human induced pluripotent stem cells (iPSCs) offer great potential for the future of medicine. We look into the world of human iPSCs, exploring their incredible promise and the critical significance of directing their development towards specialised cell lineages.
iPSC Development: Technical, Clinical, and Regulatory Hurdles
Induced pluripotent stem cells hold immense promise in revolutionising medicine through patient-specific therapies. However, their clinical development presents intricate challenges, including safety validation, differentiation complexities, and ethical considerations.
Exploring the Impact of iPSC-Derived Organoids on Biomedical Science
Discover the role of iPSC-derived organoids in biomedical science, advancing our understanding of human biology and opening doors.
The Ethical Advantages of iPSCs
Stem cell research has long been mired in ethical controversies, primarily around embryonic stem cell use, which involves complex moral and philosophical debates. The advent of iPSCs presents a turning point, promising a less contentious path forward in regenerative medicine.
IPSCs, what are they? And what is their potential?
What are iPSCs? And how can we harness induced Pluripotent Stem Cells’ potential for the betterment of patient care?
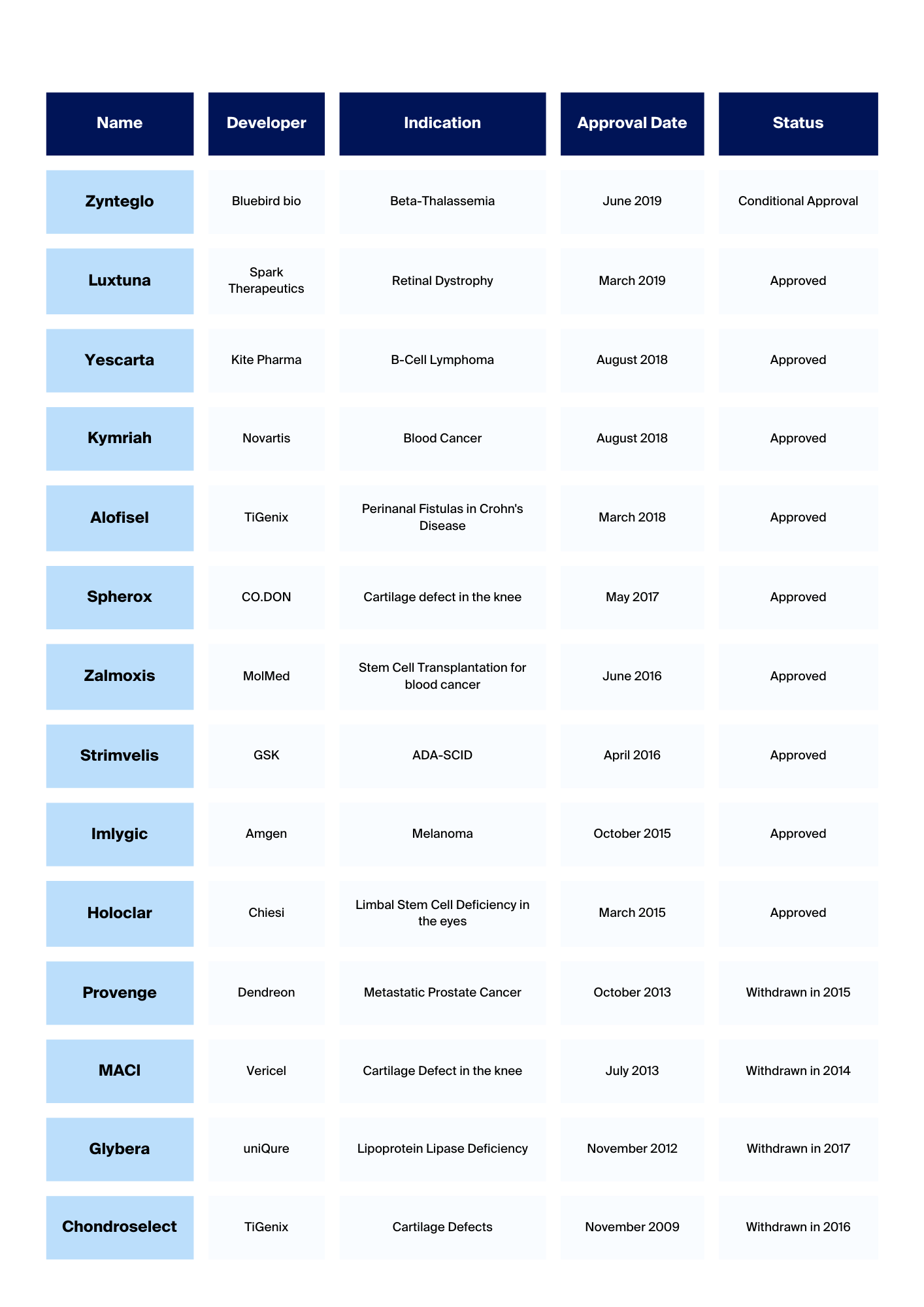
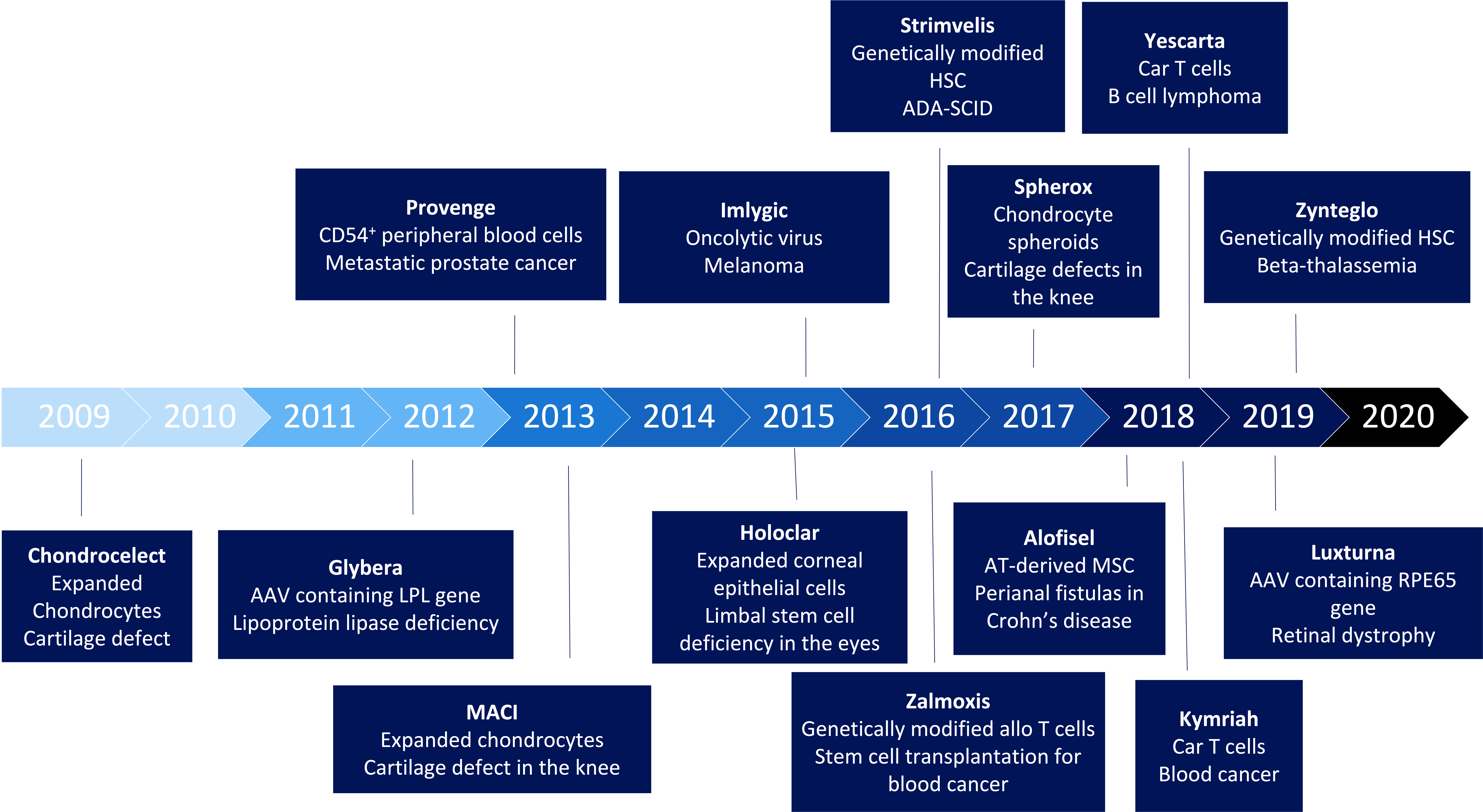
Cell and Gene Therapy companies in Europe
European Cell & Gene Therapy CompaniesAt NecstGen, we aim to support industry partnerships and growth. And to enable the next generation of therapies, it is pivotal to have oversight of the gene and therapy landscape to start from, which is why we created this...
Outsourcing Cell Therapy Manufacturing
Here are the questions you want to ask yourself when considering the outsourcing Cell Therapy Manufacturing to a CDMO. Do you have additional questions? Let us know!
NecstGen participates in € 300 million stem cell consortium
Today, the Novo Nordisk Foundation announced the € 300 million funding of an international consortium including Leiden University Medical Center, the Danstem Institute at the University of Copenhagen and the Murdoch Childrens Research Institute in Melbourne to bring...
Ex Vivo Gene Therapy
Ex vivo gene therapy is one of the most promising areas in the fight against genetic disorders. By modifying cells outside the body and then reintroducing them into the patient, this approach ensures a high degree of control and precision.
The First Stages of CAR T Cell Therapy Development
Chimeric Antigen Receptor (CAR) T cell therapy represents a transformative approach in medicine, particularly in oncology. This method offers personalised treatment by harnessing a patient's immune system to target and eliminate (cancer) cells. These novel therapies...
Webinar LV Process for CAR T
Shifting from traditional, ad hoc methodologies to a structured, data-based development approach to create a scalable, GMP-ready process.
Webinar CAR Therapy Today & Tomorrow
Explore available CAR therapies, differentiation, development challenges, and lessons learned from our expert scientist.
NecstGen and ProteoNic Report Development of Improved Viral Vectors Through the Application of Premium 2G UNicTM Technology
NecstGen and ProteoNic announce the successful development of improved lentiviral vectors for Cell and Gene Therapy, leveraging ProteoNic’s premium 2G UNic™ technology.
Pan Cancer T and NecstGen Collaborate to Accelerate Novel TCR-T Therapies into Clinical Development
Pan Cancer T and NecstGen Collaborate to Accelerate Novel TCR-T Therapies into Clinical Development.
Cell and Gene Therapy companies in Europe
European Cell & Gene Therapy CompaniesAt NecstGen, we aim to support industry partnerships and growth. And to enable the next generation of therapies, it is pivotal to have oversight of the gene and therapy landscape to start from, which is why we created this...
ProteoNic and NecstGen establish partnership to improve viral vector manufacturing efficiency for Gene Therapies
Leiden, Netherlands, April 26, 2022 - ProteoNic, a leading provider of premium vector technology and services for efficient production of biologics, and NecstGen, a CDMO and centre of excellence for Cell and Gene Therapy, announce a partnership for the development of...
European Landscape For Cell & Gene Therapy companies
At NecstGen, we aim to support industry partnerships and growth. And to enable the next generation of therapies, it is pivotal to have oversight of the gene and therapy landscape to start collaborations and partnerships so we can challenge today’s possibilities and enable the unthinkable, together.
Did you find what you were looking for?
If not, we would be happy to answer any questions you might have regarding our vacancies or life at NecstGen.