The Complexities of iPSC Development
Induced pluripotent stem cells hold immense promise in revolutionising medicine through patient-specific therapies. However, their clinical development presents intricate challenges, including safety validation, differentiation complexities, and ethical considerations to consider.
The Potential Applications of iPSC Therapies
Induced pluripotent stem cells (iPSCs) represent a significant breakthrough in regenerative medicine, heralding new possibilities for treating diseases once thought incurable.
These versatile cells can potentially treat various health conditions and provide insights into disease mechanisms. The unique characteristic of induced pluripotent stem cells (iPSCs) is that they can be derived from a patient’s cells, enabling researchers to develop disease models. This advances more precise drug testing and the formulation of personalised treatment strategies.
iPSCs are also revolutionising personalised medicine, enabling tailored regenerative therapies and advancing research and development. They facilitate the creation of patient-specific cells for repairing damaged tissues, studying disease, and developing immunotherapies, thus heralding a new era of targeted and effective medical interventions.
The Technical & Scientific Challenges of iPSCs
While iPSC-based therapies may offer unprecedented opportunities for some of the applications listed above, there are plenty of technical challenges to consider.
The Genetic Integrity Conundrum
Reprogramming somatic cells into iPSCs can introduce unwanted mutations. These genetic aberrations can compromise the functionality and safety of iPSC-derived tissues.
For researchers, the challenge is finding a way of maintaining genetic fidelity throughout the iPSC generation process, while still navigating the need for rigorous quality control measures to minimise the risk of introducing detrimental mutations.
Proliferation and Teratoma Risk
A unique property of iPSCs is their ability to proliferate indefinitely (self-renewal), yet this self-renewal capacity can become a double-edged sword when iPSCs are used for transplantation.
The risk of teratoma formation is concerned with using cells derived from induced pluripotent stem cells (iPSCs), not with the iPSCs themselves. When iPSCs are differentiated into specific cell types before implantation, the challenge lies in ensuring these cells do not proliferate uncontrollably. Balancing cell expansion to prevent unwanted growth is critical to developing safe iPSC-based therapies.
Integration & Immuno-compatibility
Successfully delivering iPSC-derived cells to target tissues and ensuring their integration within the host environment present substantial hurdles.
Precise delivery and engraftment techniques are critical to maximise the therapeutic benefits of iPSC-based therapies. The challenge lies in achieving the correct location, timing, and interaction with the host tissue.
To compound this further, even when iPSCs are derived from a patient’s cells (autologous iPSCs), immunological responses can still occur when iPSC-derived tissues are transplanted, potentially leading to rejection.
Strategies to enhance graft survival, such as immune modulation and engineering techniques, are being developed to address this nuanced challenge.
Mastering Cellular Fates: Challenges in Directed Differentiation
Efficiently coaxing iPSCs into desired cell lineages and ensuring their subsequent maturation and functionality is complex. Differentiation protocols must be finely tuned to yield high-quality, functional cells.
Researchers face hurdles in optimising protocols, to prevent suboptimal outcomes that limit the therapeutic potential of iPSC-derived cells.
While induced pluripotent stem cells (iPSCs) possess remarkable and offer prospects for personalised treatments and tissue regeneration, several complexities surround their use. These include issues related to the lack of maturity of iPSC-derived cells, which do not consistently achieve full functionality equivalent to their adult cell counterparts. Additionally, the challenges extend to ensuring the safety, ethical considerations, and control of cell differentiation. As the scientific community progresses in addressing these challenges, iPSC-based therapies may become a cornerstone of patient-specific medical treatments.
Regulatory & Ethical Considerations
In addition to the technical and scientific challenges of iPSCs, there is also a strong need for regulatory and ethical considerations. Stringent regulations are required for approval, as are the ethical considerations that include informed consent and protecting patient well-being.
Rigorous safety assessments through each stage of clinical trials are imperative to address concerns like tumorigenicity, and immunogenicity can only be achieved through clear experimental endpoints with robust release criteria.
Ethical Minefields
Complex ethical debates emerge with the use of iPSCs. Potential modifications using iPSC-derived germ cells raise ethical questions about altering the human genome in ways that could impact future generations. These concerns highlight the need for careful consideration of the long-term effects and the ethical implications of such genomic interventions. The unpredictability of unforeseen long-term consequences associated with iPSC therapies adds further ethical dimensions.
Ethicists, researchers, and policymakers must engage in thoughtful discourse to navigate the promise of iPSCs in medicine with the responsibility to safeguard against unforeseen consequences and maintain ethical integrity.
Contrasting iPSCs with Other Stem Cell Modalities
Contrasting iPSCs with other stem cell modalities reveals a compelling comparative perspective, highlighting the distinct developmental complexities inherent in each approach.
iPSC therapies – while patient-specific and ethically sound – present challenges related to differentiation and tumorigenicity. In contrast, embryonic stem cells (ESCs) offer robust differentiation potential but come with ethical concerns regarding embryo use.
Adult stem cells (ASCs) are less versatile in differentiation but generally raise fewer ethical issues.
Potential Innovations and Solutions
To advance the potential of iPSCs, new technologies and techniques must continue to evolve with the latest research.
Next-Gen Reprogramming
Ongoing research is driving the evolution of reprogramming methods towards safer and more efficient approaches in regenerative medicine.
This next generation of reprogramming techniques focuses on enhancing safety by reducing the risk of genetic mutations and tumorigenicity associated with iPSCs. Simultaneously, researchers strive for greater efficiency, streamlining the reprogramming process to produce iPSCs more rapidly and precisely.
These advancements promise to accelerate the development of personalised therapies, minimise potential risks, and broaden the scope of iPSC-based treatments. As technology continues to advance, the future of reprogramming holds the potential to revolutionise regenerative medicine.
Synergy with Advanced Therapeutics
Integrating iPSCs with therapies like CRISPR-based gene correction opens a realm of unprecedented possibilities in medicine. These synergies leverage the regenerative potential of iPSCs with the precision of CRISPR to address genetic diseases at their roots.
iPSCs can be engineered using CRISPR to correct or replace defective genes, offering patient-specific, customised treatments. This approach holds immense promise for previously incurable genetic disorders.
Furthermore, iPSCs can serve as a renewable source for generating cells for transplantation, enhancing the safety and efficacy of cell-based therapies. The convergence of iPSCs and CRISPR represents a groundbreaking frontier, propelling medicine towards more precise, effective, and personalised therapeutic interventions.
Conclusion
The promise of iPSC therapies is set to revolutionise scientific and medical applications. iPSCs offer hope for countless individuals suffering from diseases once deemed untreatable, signalling a future where personalised medicine could become the standard, not the exception.
Yet, this promise comes with its share of intricacies. The path to realising the full potential of iPSC therapies is a tapestry woven with scientific, ethical, and regulatory aspects, each adding its complexity to the challenge.
At NecstGen, we are dedicated to accelerating safe and effective cell and gene therapy applications. To learn how we can help with your development or manufacturing of stem cell and gene therapies, contact us to discuss your challenges.
Related Questions
Which Cell Therapies are approved?
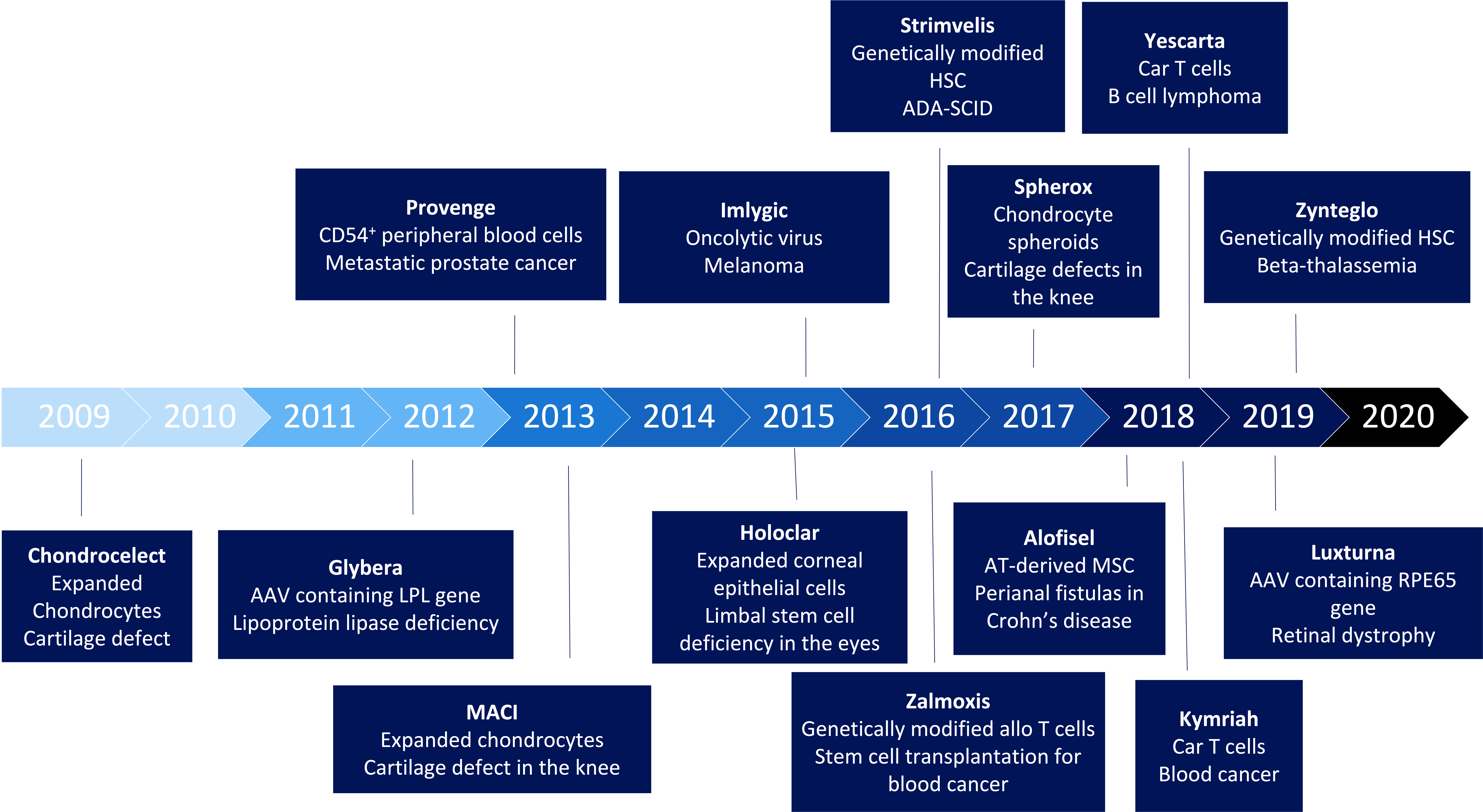
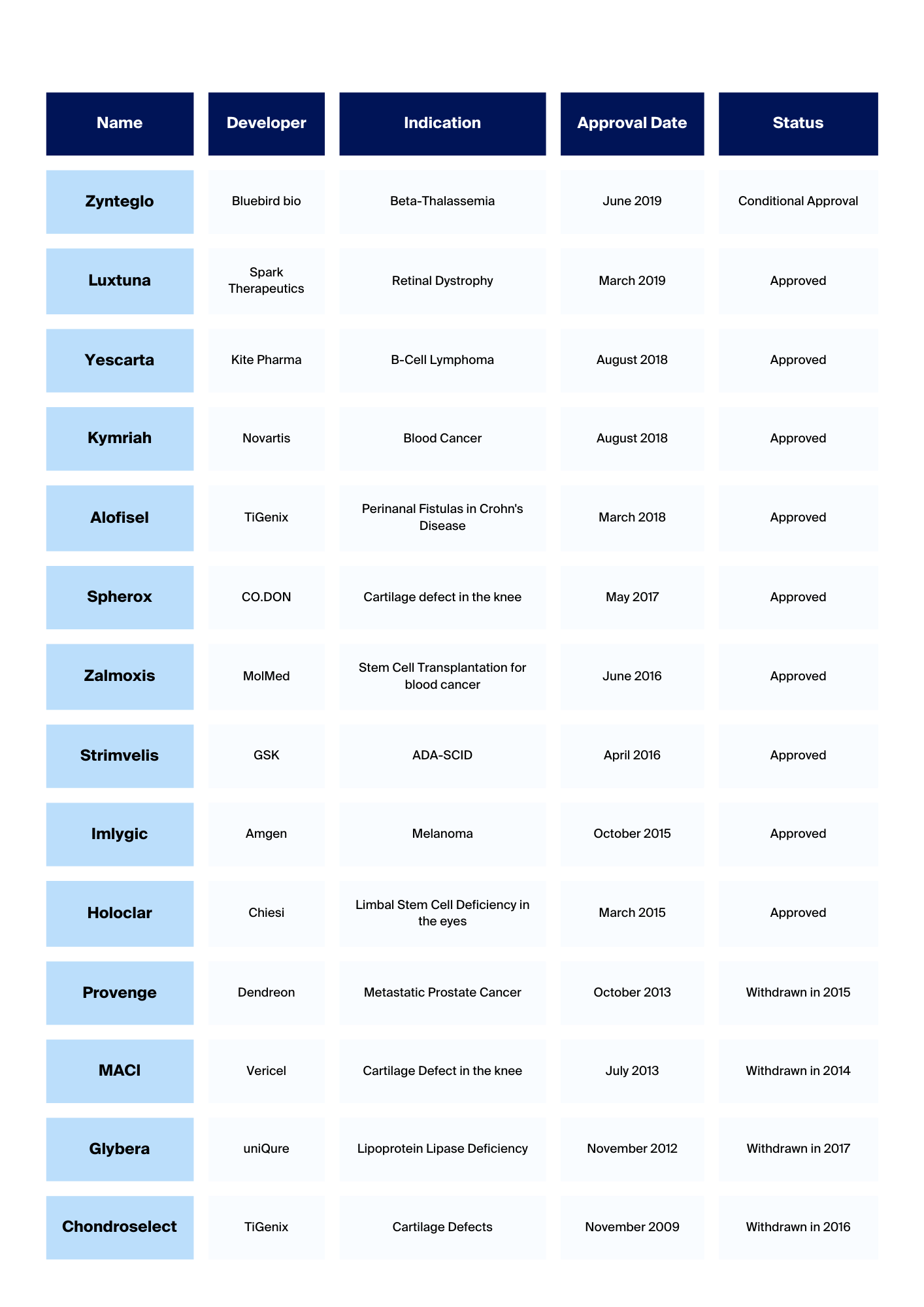
In these figures, we gathered and visualised overviews of approved ATMPs over the past years for you.

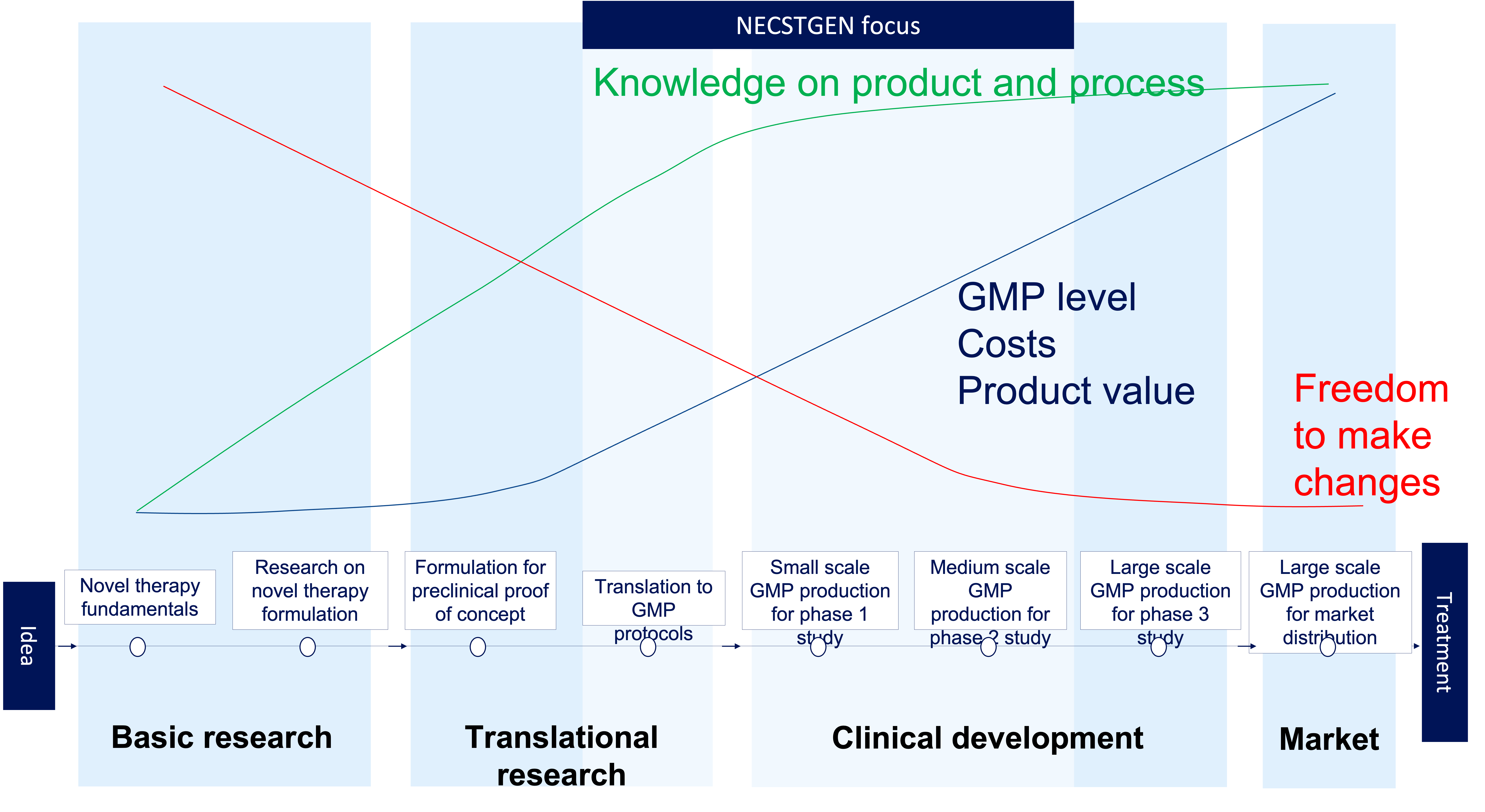
What does the Cell Therapy Development process look like?
From idea to treatment, you’ll face changing requirement and development challenges. View the figure to see how knowledge of the process inversely relates to freedom to make changes to your process.

Our experts are only a message away to help you understand the impact of any of these aspects and make informed decisions on outsourcing.
We’d be happy to discuss and help you bring cell therapies to patients.