Exploring the Impact of iPSC-Derived Organoids on Biomedical Science
Organoids have surged to the forefront of biomedical research, revolutionising the field with iPSC technology. These 3D cellular models hold transformative potential for medical breakthroughs and research, propelling our understanding and treatment of complex diseases to unprecedented heights.
What are Organoids?
Organoids are three-dimensional cell cultures that closely replicate the complex structure and functionality of real organs, bridging traditional two-dimensional cell cultures and living organisms. These miniature, simplified versions of organs are cultivated from stem cells—either pluripotent or organ-specific progenitor cells—that have the extraordinary ability to differentiate into multiple cell types.
The true innovation of organoids lies in their three-dimensional structure, which is essential for cells to interact in a manner that closely resembles their natural environment in the body. This spatial configuration allows the cells to organise themselves into complex, organ-like structures that exhibit multiple functions as human organs, such as contracting like heart tissue or forming neural networks like the brain.
Organoids can be generated to model several organs, including the brain, intestine, liver, kidney, and even the retina. This technology provides a versatile platform for scientists to study a vast array of biological processes and diseases in a controlled setting. This technology is up-and-coming for personalised medicine; organoids derived from a patient’s cells can be used to test how they might respond to different treatments, providing a tailored approach to therapy.
Organoids stand at the confluence of current research and future medical breakthroughs, embodying the promise of what science can achieve when it replicates and harnesses the intrinsic capabilities of human cells.
Deriving Organoids from iPSCs
Deriving organoids from iPSCs is a process derived from the ability of iPS cells to differentiate into any cell type.
iPSCs are coaxed into becoming organoids through a series of carefully orchestrated steps. These begin with the reprogramming of adult cells into iPSCs, followed by exposure to specific signaling cues that guide their development into organ-specific cells.
Researchers use precise combinations of growth factors and 3D culture techniques to encourage iPSCs to form structures that resemble mini-organs, complete with multiple cell types and complex organ-like functionality.
The Development of iPSC Organoids
The growth factors and cell culture media used in this process are pivotal in ensuring that iPSCs differentiate into specific types of organoid structures and are used to mimic the cellular signals present during organ development in an organism.
In addition to growth factors and media, scaffolds and matrices provide a 3D framework that offers a substrate for the cells to support the iPSC-derived cells as they grow and organise.
The Advantages of Using iPSC-Derived Organoids
iPSCs have revolutionised the field of regenerative medicine, offering unprecedented opportunities for personalised medicine, disease modelling, drug discovery, and the potential for organ transplantation. Here, we delve into the multifaceted advantages of using iPSC-derived organoids in medical science.
Personalised Medicine
iPSC organoids, which carry the genetic makeup—and potentially the same disease markers as the donor—allow for highly individualised treatment strategies. Physicians can use these organoids to test various drug responses, tailoring treatments specific to the individual’s cellular profile. Such a customised approach could significantly enhance treatment efficacy and minimise adverse effects, opening a new era of patient-centric therapy.
Disease Modeling
Researchers can replicate disease processes in a controlled laboratory environment by coaxing iPSCs to form organoids that mimic the complexity of human organs. This allows for a deeper understanding of disease pathogenesis at a cellular and molecular level and makes it possible to study with greater precision, potentially revealing novel therapeutic targets.
Drug Discovery and Toxicity Testing
Organoids provide a more accurate human tissue model for testing the efficacy and safety of new drug compounds, reducing the reliance on animal testing, which often fails to translate to human biology. Furthermore, organoids can help identify toxic side effects early in the drug development process, reducing the costs associated with late-stage drug failures and, more importantly, improving the safety profile of new medications.
Organ Transplantation Potential
Since organoids are derived from a patient’s cells, they could theoretically be used to grow transplantable tissues that are fully compatible with the recipient, virtually eliminating the risk of rejection. While this application is still largely in the research phase, it promises a future where organ shortages are no longer a concern and transplant patients can receive bespoke organs with significantly reduced complications.
Challenges and Limitations of iPSC Organoids
Despite their vast potential, some inherent challenges and limitations must be navigated to harness their total scientific and therapeutic value.
Incomplete Organ Mimicry
iPSC organoids are a monumental step towards replicating human organ structure and function in vitro. However, these miniaturised organ models do not fully recapitulate their full-sized counterparts’ complex architecture and functionality.
Organoids often lack the complete array of cell types found in actual organs, and they typically do not replicate the intricate organ-specific microenvironments, vasculature, and innervation. This incomplete mimicry limits their use as accurate physiological replicas for studying complex organ behaviours or organ replacement therapies.
Variability & Standardisation
Another significant hurdle is the high degree of variability observed in iPSC organoid cultures. Factors such as differences in iPSC lines, culture conditions, and organoid generation protocols can lead to inconsistencies in size, shape, and cellular composition.
This variability poses a challenge for standardisation, which is essential for research reproducibility and the potential clinical application of organoids. Developing standardised protocols and benchmarks for organoid generation is crucial to ensure the reliability and comparability of results across studies.
Ethical Considerations
iPSC organoid research also raises ethical concerns, particularly regarding brain organoids. As brain models become more complex and better able to recapitulate aspects of the central nervous system, questions arise about the potential for consciousness or pain perception.
This concern is especially pertinent when organoids exhibit neural activity patterns akin to those of preterm human brains. The ethical implications of creating living models of human organs, the management of patient-derived tissues, and the potential for organoid use in transplantation also raise important questions about consent, the definition of life, and the moral status of these entities.
Final Thoughts
iPSC organoids herald a new era in medical science, blending the promise of personalised medicine with the rigours of innovative research. These complex 3D cultures mirror the human body more accurately than ever before, offering a dynamic tool for disease modelling, drug discovery, and the prospect of customised organ transplantation.
Yet, the path to successfully implementing organoids is met with many challenges.
For companies looking to navigate the complexities of iPSC organoids, NecstGen can help develop or manufacture stem cell and gene therapies. Reach out to our team, and we will be happy to discuss your challenges.
Related Questions
Which Cell Therapies are approved?
In these figures, we gathered and visualised overviews of approved ATMPs over the past years for you.

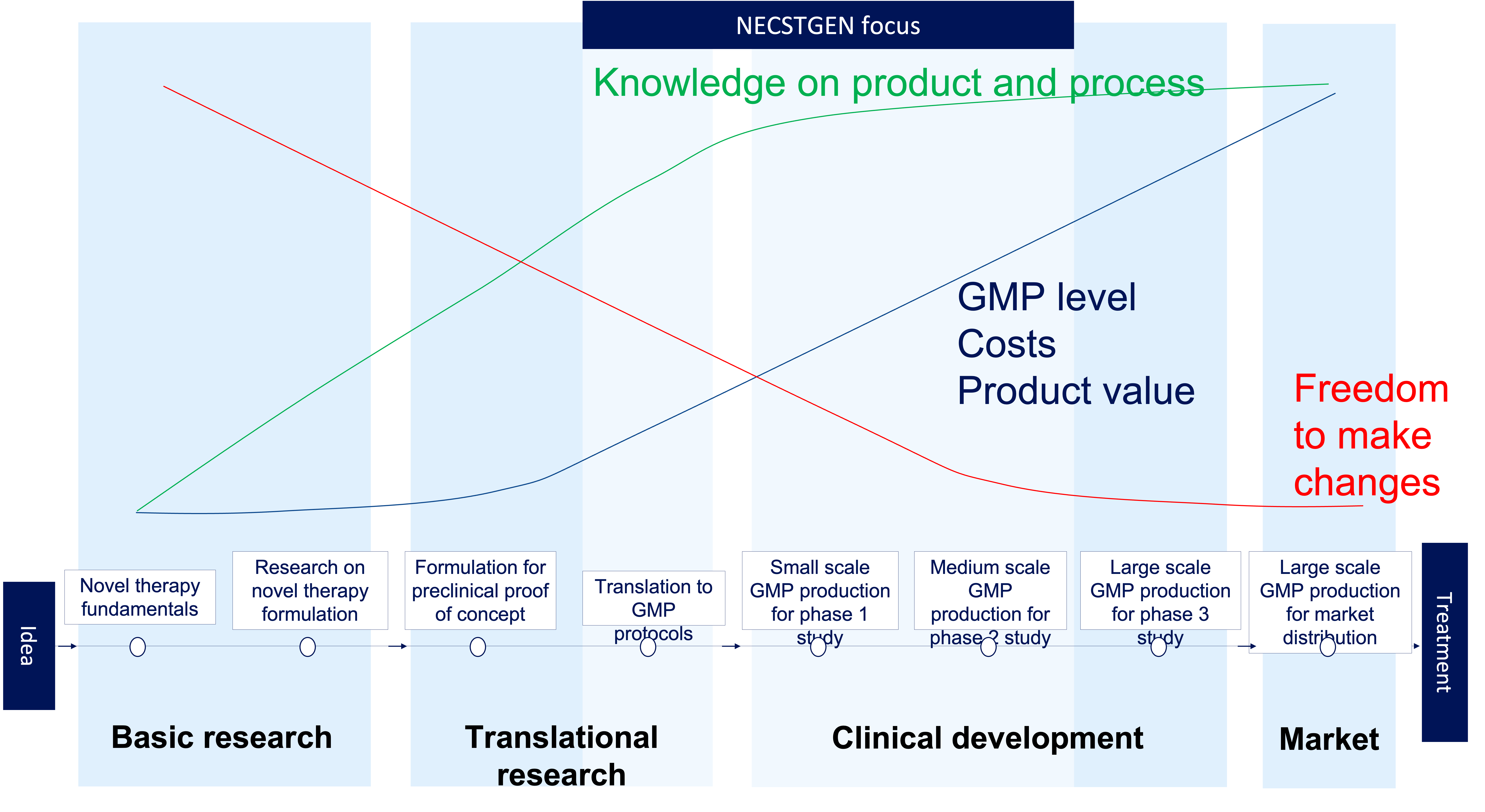
What does the Cell Therapy Development process look like?
From idea to treatment, you’ll face changing requirement and development challenges. View the figure to see how knowledge of the process inversely relates to freedom to make changes to your process.

Our experts are only a message away to help you understand the impact of any of these aspects and make informed decisions on outsourcing.
We’d be happy to discuss and help you bring cell therapies to patients.