Unlocking Cellular Potential With iPSC Reprogramming and Cell Transdifferentiation
iPSC reprogramming and transdifferentiation both offer exciting opportunities in cell biology. Learn how these approaches are revolutionising regenerative medicine and cell therapy.
What is Cellular Reprogramming?
Cellular reprogramming is a process by which an adult, specialised somatic cell is transformed into a pluripotent state—an iPSC (induced pluripotent stem cell). These stem cells can differentiate into any cell in the body.
As such, cellular reprogramming presents new opportunities in personalised medicine, disease modelling, and tissue regeneration and represents a monumental step towards a future in which we can alter cellular identities to combat diseases and enhance human health.
What Are Induced Pluripotent Stem Cells (iPSCs)?
iPSCs are stem cells reprogrammed from fully differentiated cells, such as skin or blood cells. While the ability of hiPSCs to differentiate into virtually any cell type is a fundamental characteristic, it’s important to distinguish this from the actual process of hiPSC reprogramming. Reprogramming refers to the initial conversion of differentiated adult cells into pluripotent stem cells. The differentiation of these reprogrammed cells into various cell types, similar to what embryonic stem cells can do, is a separate phase that follows the reprogramming. This distinction is crucial for understanding the scope and potential applications of hiPSC technology in research and clinical settings.
The Advantages & Limitations of iPSCs
iPSCs have generated interest in stem cell research for several reasons:
Origin from Patient-Specific Cells: hiPSCs can be derived from an individual’s cells, reducing the risk of immune rejection when used for transplantation.
Disease Modelling: Researchers can create hiPSCs from patients with genetic disorders or diseases and study disease mechanisms at the cellular level to screen potential drug candidates.
Regenerative Medicine: By differentiating into tissue/organ-specific cell types, hiPSCs hold the potential to replace damaged or malfunctioning tissues and organs, providing tailored solutions for patients.
Ethical Advantages: iPSCs circumvent some ethical concerns associated with embryonic stem cells, as they do not require the destruction of embryos for their generation.
While iPSCs offer numerous advantages in stem cell research and regenerative medicine, they also come with certain disadvantages and challenges that need to be addressed.
Tumorigenic potential: Tumorigenic potential in iPSCs is linked to genetic and epigenetic memory and differentiation efficiency. Incomplete differentiation, where some cells remain undifferentiated, increases the risk of tumour formation. To mitigate these risks in iPSC-based therapies, it’s crucial to ensure complete and efficient differentiation.
Genetic & Epigenetic Variability: Although we already discussed genetic and epigenetic modifications previously, it’s important to clarify that such changes can arise during the reprogramming process, during subsequent cell culture, or even pre-existing in the donor somatic cells. Each source contributes uniquely to the variability observed in iPSCs, impacting their behaviour and differentiation capacity.
Inefficiency & Variability: Generating iPSCs can be inefficient, with a relatively low success rate in some cases.
Immunogenicity: While hiPSCs generated from a patient’s cells can reduce the risk of immune rejection, there may still be immune responses against hiPSC-derived cells sometimes induced for ex-vivo cell culture
Time & Cost-Intensive: The generation and characterisation of iPSCs are time-consuming and costly processes.
Ethical Considerations: Although reprogrammed cells are an ethical alternative to embryonic stem cells, there are still ethical considerations related to their use, specifically involving manipulating human genetic material.
iPSC Reprogramming Techniques
Viral Vector-based: Viral vector-based reprogramming can involve various types of viruses. While lentiviruses and retroviruses can integrate reprogramming factors into the host cell’s genome, raising concerns about genomic integration and tumorigenicity, adenoviruses are also used in reprogramming. Importantly, adenoviruses do not integrate their genetic material into the host genome, potentially reducing these risks.
mRNA-based: mRNA-based reprogramming uses synthetic messenger RNA (mRNA) to deliver reprogramming factors in a non-integrative manner, which is safer in terms of avoiding genome alteration. Although this method typically involves transfection, mRNA can be introduced into cells through other techniques, broadening its application. However, it is generally considered less efficient than viral methods.
Protein-based: Direct delivery of reprogramming factors as proteins overcome genomic integration concerns, but it may require optimisation to enhance reprogramming efficiency.
Small Molecule-based: Small Molecule-based reprogramming involves using small molecules that can mimic the functions of reprogramming factors, offering an alternative strategy to induce pluripotency. While this approach is less well-established than other methods, it reduces complexity. However, it’s important to note that these small molecules can induce reorganisation of the host genome, which may have safety implications.
The choice of delivery method depends on the specific goals of the research or clinical application, and factors such as efficiency, safety, and potential genomic alterations must be considered.
What is Transdifferentiation?
In contrast to iPSC reprogramming, which involves reverting a specialised cell type to a pluripotent state before differentiating it into another type, transdifferentiation consists of redirecting one specialised cell type directly into another, bypassing the pluripotent stage entirely.
Transdifferentiation is initiated by a combination of changes in gene expression patterns, including specific transcription factors, signalling pathways, and epigenetic modifications. These factors work together to redirect a specialised cell type into another without reverting to a pluripotent state.
The Key Differences Between iPSC Reprogramming and Transdifferentiation
iPSC reprogramming and transdifferentiation are two distinct approaches in cellular biology, each with notable differences. As summarised in this image in Nature, these methods alter cell states, specifically geared towards applications in regenerative medicine and cellular therapy.
iPSC reprogramming involves converting a differentiated (adult) cell, such as a skin cell, into a pluripotent stem cell.
Transdifferentiation, or direct reprogramming, takes a different approach. It involves converting one type of adult cell directly into another without the need for a pluripotent stage.
Recognising Challenges & Future Prospects
Both iPSC reprogramming and transdifferentiation methodologies have made remarkable progress in a short amount of time, but still face challenges. The current limitations of iPSCs and transdifferentiation include the risk of tumorigenicity, genetic and epigenetic variability, and the need for further optimisation to enhance efficiency.
Future prospects for iPSC reprogramming and transdifferentiation are promising due to ongoing advancements in understanding the underlying molecular mechanisms and improving the technologies. Researchers are continually developing safer and more efficient methods, which could lead to breakthroughs in personalised medicine, disease modeling, and regenerative therapies. As these techniques become more refined, their potential to transform medical treatments and outcomes becomes increasingly feasible.
The integration of emerging technologies and innovative approaches will undoubtedly continue to shape the future of these transformative fields.
Conclusion
The remarkable ability to alter a cell’s identity through induced reprogramming and transdifferentiation has ushered in a new era of science.
However, it must also recognise the challenges that lie ahead. We can overcome these obstacles through collaboration, dedication, and continued advances and fully harness the potential of cellular reprogramming.
At NecstGen, we are at the forefront of pioneering CGT research. To learn how we can help with your development and manufacturing of stem cell and gene therapies, reach out to discuss your challenges.
Related Questions
Which Cell Therapies are approved?
In these figures, we gathered and visualised overviews of approved ATMPs over the past years for you.

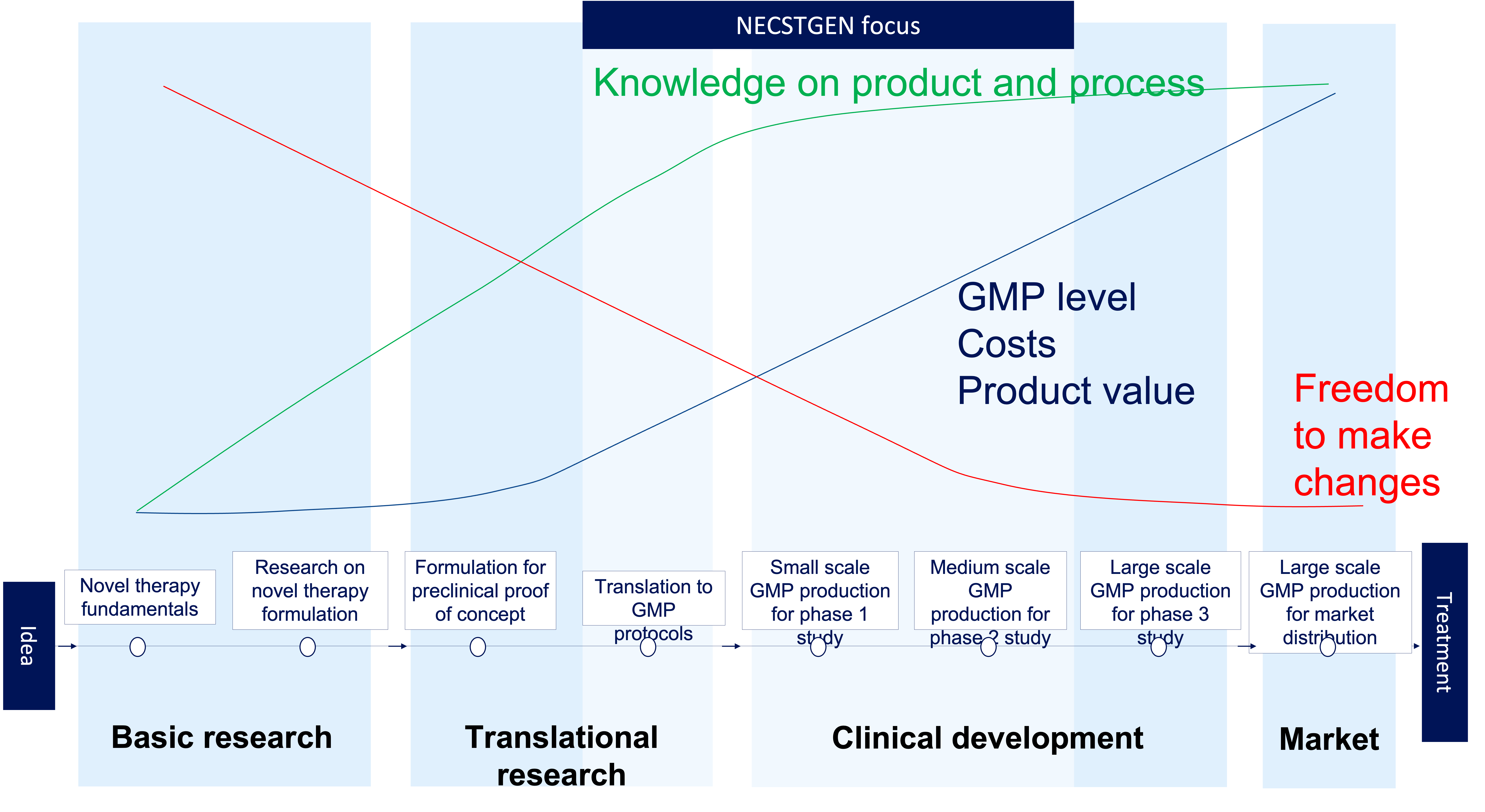
What does the Cell Therapy Development process look like?
From idea to treatment, you’ll face changing requirement and development challenges. View the figure to see how knowledge of the process inversely relates to freedom to make changes to your process.

Our experts are only a message away to help you understand the impact of any of these aspects and make informed decisions on outsourcing.
We’d be happy to discuss and help you bring cell therapies to patients.