
The First Stages of CAR T Cell Therapy Development
From Discovery to Preclinical Development: What are the critical stages to look out for?
Introduction
Chimeric Antigen Receptor (CAR) T cell therapy represents a transformative approach in medicine, particularly in oncology. This method offers personalised treatment by harnessing a patient’s immune system to target and eliminate (cancer) cells. These novel therapies undergo a meticulous journey from target discovery to clinical application, starting with three fundamental steps: target identification, CAR design, and preclinical testing. Here, we want to focus on these initial steps, emphasising their crucial role in developing efficacious and safe CAR T cell therapies.
Target Identification
The basis of efficacious CAR T cell therapy is in selecting an appropriate target antigen for respective target cells. The target antigen must meet several criteria to ensure the therapy’s efficacy and safety.
Target Antigens in CAR T Cell Therapy
An antigen is any substance that causes the immune system to raise a specific immune response against it. In CAR T cell therapy, CARs are used to redirect T cells to recognise and eliminate cells expressing a specific target antigen. The target antigen must be highly specific to the target cells to avoid unwanted side effects such as the killing of healthy cells and tissues, which can be a side effect of the treatment. One significant target antigen that has been approved by the EMA (European Medicines Agency) is BCMA (B-cell Maturation Antigen). CAR T cell therapies like Abecma (Idecabtagene Vicleucel) and Carvykti (Ciltacabtagene Autoleucel) have been approved for treating relapsed or refractory multiple myeloma. These therapies modify T cells to target BCMA, which is commonly expressed on multiple myeloma cells.
(https://www.ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-risk-assessment-committee-prac-8-11-january-2024#ema-inpage-item-64904)
Antigen Selection Criteria for CAR T Cell Therapy
The ideal target antigen should be:
- Highly Expressed on Cancer Cells: Ensuring that the therapy targets most cancer cells.
- Minimally Present on Healthy Cells: Reducing the risk of off-target effects and associated toxicities.
- Uniformly Expressed Across Cancer Cells: Providing consistent targeting of all malignant cells. However, marker expression by (solid) tumours may change over time leading to tumour escape from the therapy.
Understanding the characteristics of a potential therapy, including safety, efficacy, and toxicity profile of a CAR T cell product as early as possible is essential for guiding strategic decisions and helps de-risking investment in further clinical development.
Assessing whether a specific antigen is (or is not) expressed on a specific tissue can be done using these methods:
- In Silico Analysis: Bioinformatics tools analyse gene expression data to predict potential antigens highly specific to cancer cells.
- In Vitro Studies: Laboratory experiments using cancer cell lines and patient-derived cells help confirming antigen.
- In Vivo Models: Animal studies, typically in mice, help assessing whether the antigen is expressed on a specific tissue.
Chimeric Antigen Receptor (CAR) Design
The next critical step is generating the CAR construct.
Components of a CAR
A CAR consists of four primary components:
- Extracellular Domain: Derived from a monoclonal antibody, this domain binds to the target antigen on cancer cells.
- Hinge Region: Provides flexibility to the CAR, facilitating effective binding to the antigen.
- Transmembrane Domain: Anchors the CAR to the T cell’s surface.
- Intracellular Signalling Domain: This part of the CAR includes co-stimulatory domains (CD28 or 4-1BB) and an activation domain (CD3ζ), which trigger T cell activation and proliferation upon antigen binding.
A schematic overview of a CAR can be found in the figure below.
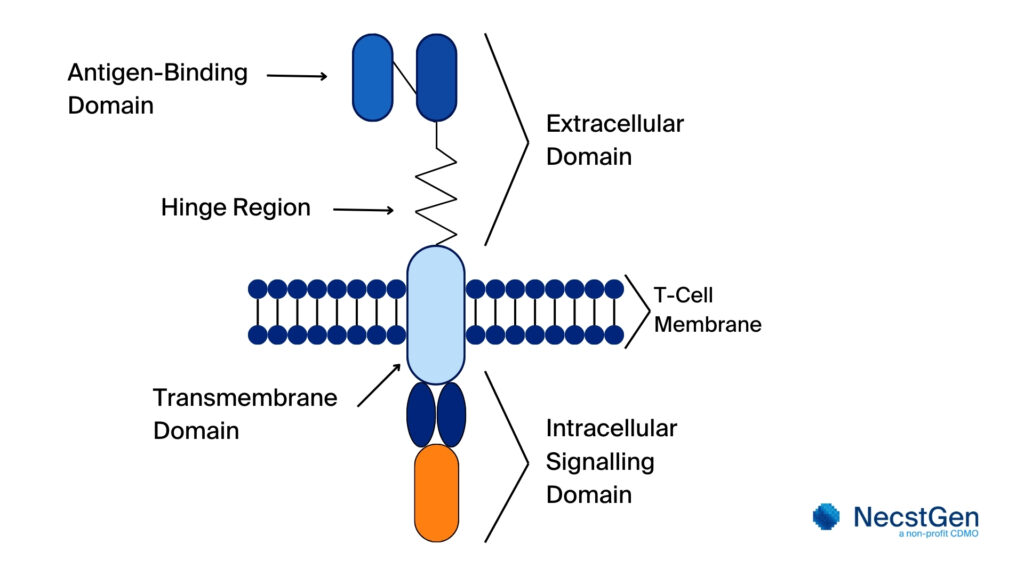
Figure 1. Schematic overview of a CAR. The schematic overview shows key components. The extracellular antigen-binding domain binds to the target antigen on cancer cells. The hinge region, a flexible segment, links this domain to the transmembrane domain, aiding positioning and motility. Within the antigen-binding domain, the extracellular domain identifies the specific antigen. The transmembrane domain anchors the CAR in the T cell membrane, while the intracellular signalling domain transmits activation signals upon antigen binding, triggering the T cell’s response.
Engineering and Optimisation of CAR T Cells
The process of engineering and optimisation of a CAR begins with synthesising the CAR gene. This gene is then incorporated into for example a viral vector, often a lentivirus or retrovirus. Introducing the CAR gene into T cells by using a viral vector is called viral transduction. The engineered CAR is then tested in cell lines to confirm its functionality. The next step is the optimisation of the CAR T cells, which includes but is not restricted to:
- Affinity Tuning: Adjusting the binding strength of the CAR to the antigen to achieve a balance between efficacy and safety.
- Signal Modulation: Enhancing the T cell’s response to ensure effective cancer cell destruction without causing excessive activation that could lead to toxicity.
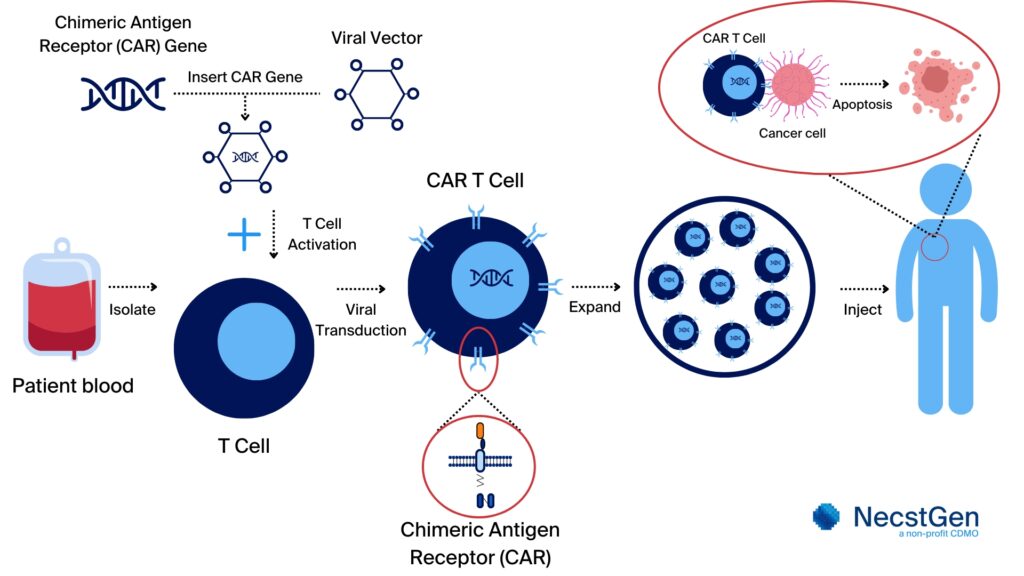
Figure 2. Overview of CAR T cell therapy development. The process starts with harvesting peripheral blood cells from the patient. Then, mononuclear cells are isolated from the blood. If needed, the T cells are isolated from the mononuclear cells. These (isolated) T cells are then genetically modified to express chimeric antigen receptor (CAR) that target specific (cancer) cells. Following activation of the T cells, [Mv1] the insertion of the CAR gene is performed using a viral vector (viral transduction) or non-viral transfection methods. The modified T cells are expanded to clinically relevant numbers. Only after completing quality control testing and certification by a Qualified Person (QP), the CAR T cells can be administered to the patient, providing for a targeted and personalised treatment.
Preclinical Testing
Preclinical testing is used for the evaluation of CAR T cells in controlled environments to ensure their efficacy and safety before proceeding to a first in human clinical trial. The efficacy and safety of CAR T cells are tested using in vitro and in vivo methods.
In Vitro Testing
During in vitro testing, various standard assays are performed to understand more about the characteristics of the engineered CAR T cell, such as its cytotoxicity, proliferation, and cytokine secretion levels.
- Cytotoxicity Assays: Assessing the CAR T cells’ ability to kill target cells and to leave non-target cells untouched.
- Proliferation Assays: Measuring the expansion capacity and survival of CAR T cells upon activation by the target antigen.
- Cytokine Secretion Assays: Evaluating the secretion of cytokines as an indication for T cell activation and potential toxicity.
In vivo Testing Following successful in vitro testing, CAR T cells are evaluated in vivo using clinically relevant animal models:
- Toxicity Studies: These studies assess potential side effects and determine the maximum tolerated dose, which is a challenge as the weight of a mouse is only about 25 grams.
- Efficacy Studies: Animal models are used to test the CAR T cells’ ability to effectively reduce the tumor burden and improve survival. The safety profile and mechanism of action of the CAR T cell therapy are being assessed.
Regulatory Compliance
Preclinical studies must comply with Good Laboratory Practice (GLP) standards to ensure reliability and reproducibility of the experiments. Comprehensive documentation of findings is crucial for future regulatory submissions, such as an Investigational Medicinal Product Dossier (IMPD) application.
As an academic institution or company aiming to develop a CAR T cell therapy, collaboration with a Contract Development and Manufacturing Organization (CDMO) can be invaluable. CDMOs offer specialised expertise, resources, and capacity, that contributes to streamline the development process, enhance scalability, and ensure regulatory compliance.
Conclusion
The first stage of CAR T cell therapy development, encompassing target identification, CAR design, and preclinical testing, is fundamental in contributing to the therapy’s safety and efficacy. By meticulously planning and executing each step, therapy developers aim to bring safe and effective CAR T therapies from the laboratory to the patient.



