European Innovation Council Awards €2.5 Million Grant to Trince, NecstGen, and IBSAL for Advancing Cell Therapy Manufacturing
[Ghent, Leiden, Salamanca, 15/03/2024] – Following a rigorous selection process by an expert panel, Trince, NecstGen, and IBSAL are proud to announce that their “Penphomet” project has been selected by The European Innovation Council (EIC) for a significant grant of €2.5 million. Out of 257 eligible submissions, Penphomet is one of the 27 that has been awarded. Led by Trince, the consortium aims to revolutionize cell therapy manufacturing by integrating nanotechnology, optics, and microfluidics.
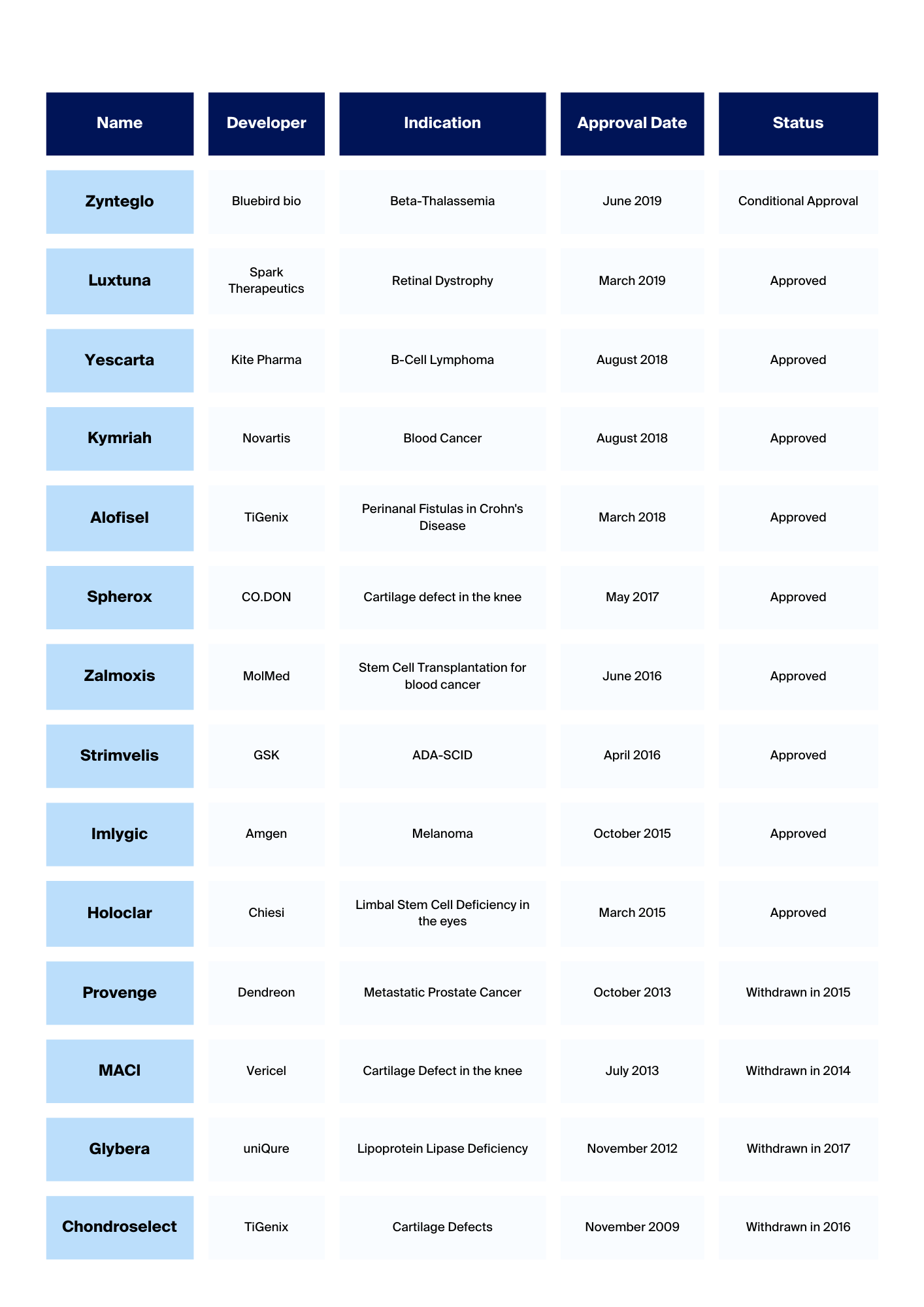
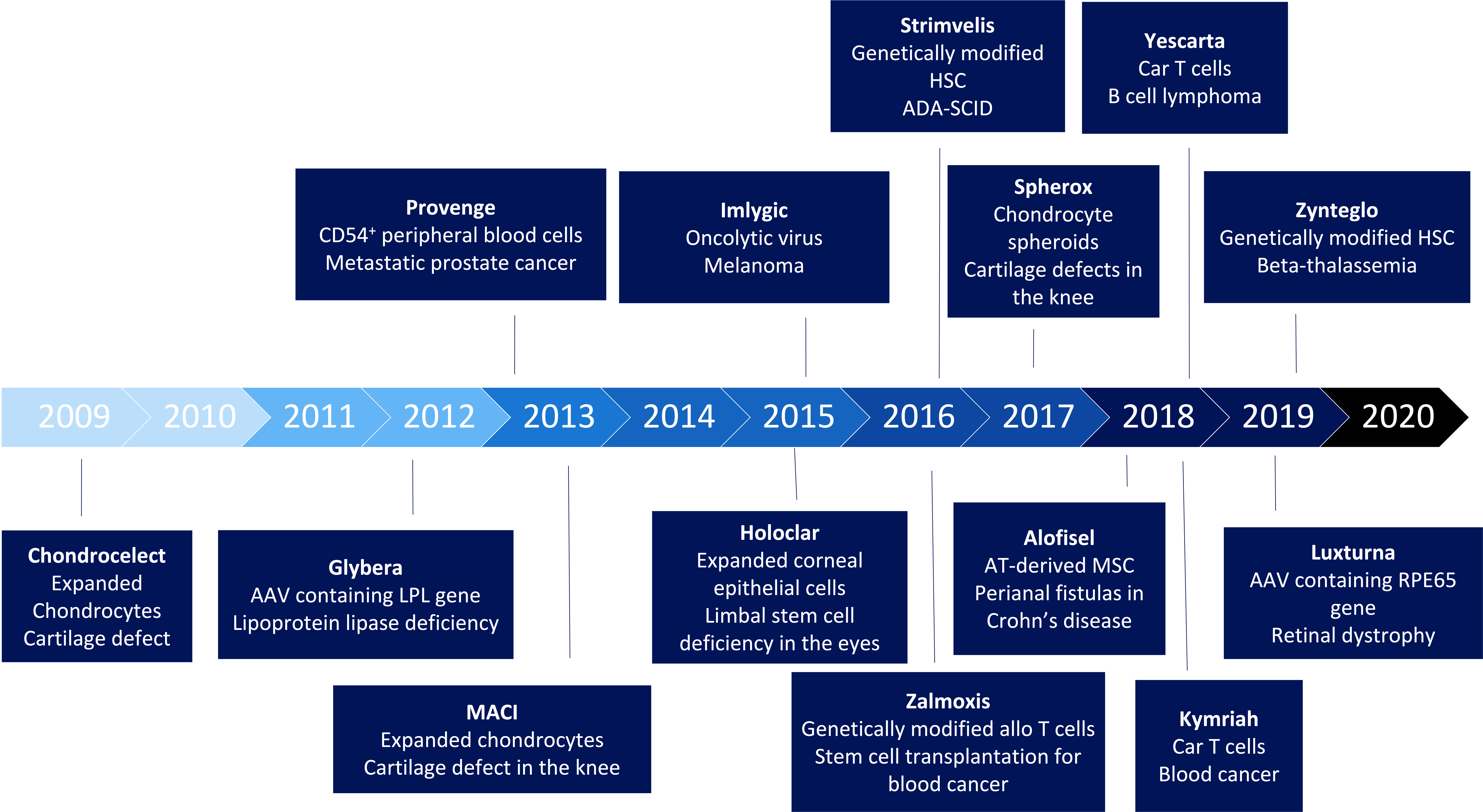
Cell therapy has shown promising results in cancer treatment, specifically using patient-derived cells like T cells and mesenchymal stromal cells (MSCs) that have been genetically engineered to effectively target cancer cells.
The primary focus of the Penphomet project is to develop a safer, non-viral method for cell engineering that minimally impacts cell functionality and phenotype. The aim is to significantly reduce the costs associated with cell therapy manufacturing.
Over three years, the project aims to deliver a fully automated, high-throughput system that can be installed in centralized cell production facilities or integrated into point-of-care cell manufacturing equipment.
“We are grateful for the EIC’s support in funding the Penphomet project. It demonstrates that our breakthrough technology is addressing a crucial gap in the field of cell therapy,” said Philip Mathuis, CEO at Trince. “Together with our partners NecstGen and IBSAL, we are committed to advancing cell therapy manufacturing, ultimately benefiting patients and healthcare systems.”
“NecstGen is proud to be a part of the Penphomet consortium supporting the further development of the innovative technology of Trince. Non-viral methods for cell engineering represent a potentially cost saving route for cell engineered therapies. And furthering their use is important for the field of Cell and Gene Therapy and mission of NecstGen to enable patient access” said Paul Bilars, CEO, NecstGen.
“For the IBSAL and its main partners, the University Hospital and the University of Salamanca, the Penphomet project opens the possibility of exploring a new strategy of cell modification that can be enormously attractive for the next generation of advanced therapy medical products, and we are really pleased to be part of this initiative,” says Prof. Fermin Sanchez-Guijo, principal investigator of the IBSAL in this project.
The Penphomet project represents a significant advancement in improving the accessibility and affordability of cell therapies, with the potential for far-reaching impacts on cancer treatment and beyond.
For media inquiries or further information, please contact:
[Philip Mathuis]
[Trince]
[+32 9 273 56 25]
[info@trincebio.com]
Trince
Trince offers a unique intracellular delivery (transfection) technology for the life sciences/biotech field. The LumiPore platform is based on the interaction between pulsed laser light and photothermal nanomaterial. By irradiating the proprietary nanoparticles with laser light, highly localized light-induced thermal and mechanical forces are generated. When these forces come into contact with the cell membrane, they create temporary pores through which external effector molecules can enter the cell. This ‘photoporation’ technology was developed as a next-generation intracellular delivery platform for efficient, flexible gentle, and safe delivery of a wide variety of effector molecules in a broad range of primary and hard-to-transfect cells, while maintaining high therapeutic quality. The technology can deliver a diverse set of payloads in various hard-to-transfect cell types, including suspension and adherent cells (directly in a standard lab recipient) and even living tissue slices.
NecstGen
NecstGen is a non-profit CDMO and centre of excellence for Cell and Gene Therapy, located in a purpose-built GMP facility on the largest bio-cluster in the Netherlands, Leiden Bio Science Park. NecstGen provides critical contract development, manufacturing and rental services to academic and industrial therapy developers to deliver a new generation of therapies to patients.
NecstGen offers:
- Full contract manufacturing services for Cell Therapy and Viral Vector
- Process design, scale-up, optimisation and automation for Cell Therapy and Viral Vector
- Assay development for in-process, release, and potency
- Cleanroom rental including services for QA, QC, and QP.
IBSAL
The Institute of Biomedical Research of Salamanca (IBSAL) is part of IECSCYL (The Fundación Instituto de Estudios de Ciencias de la Salud de Castilla y León) and is one of the Biomedical Research Institutes accredited by the Carlos III Institute from the Spanish Ministry of Health (Order of February 17, 2014). IBSAL’s mission is to develop clinical and translational research, promoting synergy between clinical and basic research groups and optimizing resources through shared services and efficient management structures. One of the 6 areas of the Institute is Gene, Cell and Transplant Therapy, coordinated by Prof. Sánchez-Guijo. The location where the project tasks will be carried out is the Hematology Department of the University Hospital of Salamanca (HUS), also chaired by Prof Sanchez-Guijo. The Department provides services a.o. in cell therapy, includes a GMP Facility and a Translational Research Lab, and has extensive experience in the preclinical and clinical development and management of ATMPs, especially MSCs but also CAR T cells.
EIC Transition projects focus on results generated by EIC Pathfinder, FET (Future and Emerging Technologies – as the predecessor of EIC Pathfinder) or European Research Council (ERC) Proof of Concept projects, to mature the technologies and build a business case for specific applications. Grants of up to €2.5 million are available to validate and demonstrate technology in application-relevant environment and develop market readiness.